Actinic keratoses started yesterday
Maybe they’re from sunning on the beach. Maybe it was those countless hours playing basketball or simply tending to your garden. No matter how you got them, actinic keratoses (AKs) are the result of prolonged sun exposure.
58 million Americans have at least one AK.
Both men and women can get crusty, scaly AK sunspots on their skin.
AKs are precancerous, and 1 in 10 may become cancerous.
Spot the spots
AKs can be as small as a pinhead or larger than a quarter. They range in color from skin toned to reddish brown and are usually dry, crusty, and scaly. They’re found in places like your face, scalp, arms, and hands. Typically, you can feel them before you can see them. Only a dermatologist can properly diagnose AKs and determine if they could become cancerous.
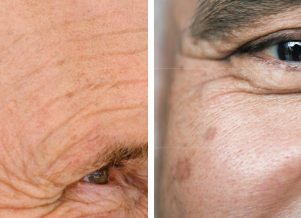
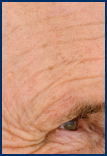
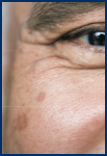
Few AKs
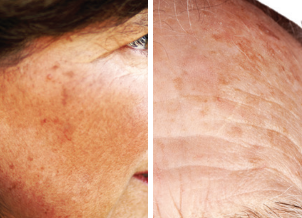
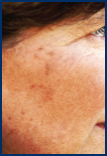
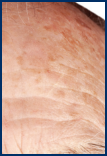
Several AKs
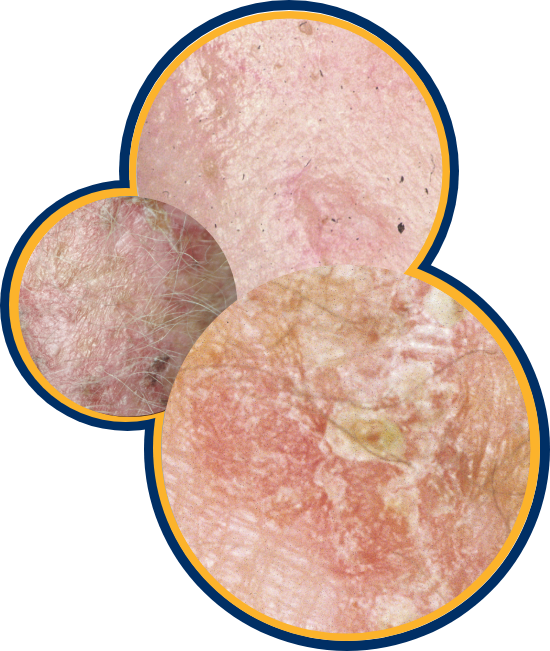

1 in 10 AKs become cancerous The sooner you treat them, the better
Find Treatment
Know your treatment options
When getting ready for your dermatologist appointment, it’s a good idea to prepare some questions about the different treatment options for actinic keratosis. If you’re not sure or you need some help, here are just a few options to talk about during your appointment:
- Photodynamic therapy (PDT)
- Topicals (ointments)
- Cryotherapy (freezing)
If you don’t want the hassle of daily topicals and are looking for a treatment for minimally to moderately thick AKs that can be done right in your dermatologist’s office without scarring, LEVULAN® KERASTICK® + BLU-U® may be a good option for you.

Give your skin a brighter future
LEVULAN KERASTICK + BLU-U uniquely combines a prescription medicine with the power of blue light therapy to treat AKs. It works faster than daily topicals, and treatment can be done right in your dermatologist’s office—with no risk of scarring.

Most AK sunspots on the face and scalp were 100% clear after 12 weeks and remained clear after 1 year of initial treatment
Patented technology can efficiently treat multiple AKs at once
No scarring was reported in clinical trials
LEVULAN KERASTICK topical solution contains alcohol and may cause skin irritation if covered or bandaged for longer than 3 hours
Steps for great results :
Bring a hat and/or a long-sleeved shirt to your appointment to cover treated skin.
IMPORTANT SAFETY INFORMATION
PATIENT INFORMATION
LEVULAN KERASTICK (aminolevulinic acid HCl) is for use only as an in-office treatment. LEVULAN KERASTICK treatment is given by a healthcare provider only and is not for use at home.
What is LEVULAN KERASTICK?
LEVULAN KERASTICK is a prescription medicine used on the skin with blue light treatment (BLU-U Blue Light Photodynamic Therapy or PDT) for the treatment of minimally to moderately thick actinic keratoses (AK’s) of the face, scalp, or upper arms.
It is not known if LEVULAN KERASTICK is safe and effective in children under 18 years of age.
Who should not receive LEVULAN KERASTICK treatment?
Do not receive LEVULAN KERASTICK treatment if you:
- are allergic to aminolevulinic acid HCl or to any of the ingredients in LEVULAN KERASTICK.
- have porphyria or are allergic to porphyrins
- have a skin sensitivity to blue light.
Before receiving LEVULAN KERASTICK treatment, tell your healthcare provider about all of your medical conditions, including if you:
- have blood clotting problems
- are pregnant or plan to become pregnant. It is not known if LEVULAN KERASTICK will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if LEVULAN KERASTICK passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby during LEVULAN KERASTICK treatment.
- Tell your healthcare provider about all of the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. LEVULAN KERASTICK and other medicines may affect each other.
How will I receive LEVULAN KERASTICK treatment?
- LEVULAN KERASTICK treatment is received in two parts:
- Your healthcare provider will apply LEVULAN KERASTICK topical solution to your skin lesions. You should not wash the treated areas before you return to your healthcare provider for blue light treatment.
- After the prescribed amount of time you will return to your healthcare provider for blue light treatment. If you cannot return for blue light treatment on schedule, avoid sunlight and bright indoor light for at least 40 hours after LEVULAN KERASTICK topical solution has been applied.
- During blue light treatment, you will likely feel tingling, stinging, prickling, or burning of the treated areas.
What should I avoid during LEVULAN KERASTICK treatment?
After LEVULAN KERASTICK topical solution is applied to your skin you should avoid sunlight or bright indoor light (such as examination lights, operating room lights, tanning beds, or lights that are close to you) for 40 hours. During this time, the treated areas of your skin will become sensitive to light.
Exposure to light during this time may cause you to feel a burning or stinging sensation and may cause your treated lesions to become red or swollen. You should wear appropriate protective apparel such as a wide-brimmed hat, long sleeve shirt, and gloves to protect your treated skin from sunlight and other bright light.
Sunscreen will not protect the treated areas of your skin against sensitivity to light.
What are the possible side effects of LEVULAN KERASTICK?
LEVULAN KERASTICK may cause serious side effects, including:
- Temporary memory problems: Temporary memory problems have happened during treatment with LEVULAN KERASTICK in combination with BLU-U Blue Light Photodynamic Therapy Illuminator. You or your family members or caregiver should call your healthcare provider right away if you develop any problems with memory, confusion, or disorientation during treatment.
- Sensitivity to light
- Skin irritation. LEVULAN KERASTICK topical solution contains alcohol and may cause skin irritation if covered or bandaged for longer than 3 hours.
The common side effects of LEVULAN KERASTICK include:
Local skin reactions including redness, swelling, stinging and burning, scaling, crusting, oozing, pustules, welts, scabbing, itching, erosion, changes in skin color, bleeding, tenderness, changes in the sense of touch, and dryness.
These are not all the possible side effects of LEVULAN KERASTICK.
Call your doctor for medical advice about side effects.
Please read full Prescribing Information and Patient Information for LEVULAN KERASTICK and discuss any questions with your doctor.
IMPORTANT SAFETY INFORMATION
PATIENT INFORMATION
LEVULAN KERASTICK (aminolevulinic acid HCl) is for use only as an in-office treatment. LEVULAN KERASTICK treatment is given by a healthcare provider only and is not for use at home.
What is LEVULAN KERASTICK?
LEVULAN KERASTICK is a prescription medicine used on the skin with blue light treatment (BLU-U Blue Light Photodynamic Therapy or PDT) for the treatment of minimally to moderately thick actinic keratoses (AK’s) of the face, scalp, or upper arms.
It is not known if LEVULAN KERASTICK is safe and effective in children under 18 years of age.
Who should not receive LEVULAN KERASTICK treatment?
Do not receive LEVULAN KERASTICK treatment if you:
- are allergic to aminolevulinic acid HCl or to any of the ingredients in LEVULAN KERASTICK.
- have porphyria or are allergic to porphyrins
- have a skin sensitivity to blue light.
Before receiving LEVULAN KERASTICK treatment, tell your healthcare provider about all of your medical conditions, including if you:
- have blood clotting problems
- are pregnant or plan to become pregnant. It is not known if LEVULAN KERASTICK will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if LEVULAN KERASTICK passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby during LEVULAN KERASTICK treatment.
- Tell your healthcare provider about all of the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. LEVULAN KERASTICK and other medicines may affect each other.
How will I receive LEVULAN KERASTICK treatment?
- LEVULAN KERASTICK treatment is received in two parts:
- Your healthcare provider will apply LEVULAN KERASTICK topical solution to your skin lesions. You should not wash the treated areas before you return to your healthcare provider for blue light treatment.
- After the prescribed amount of time you will return to your healthcare provider for blue light treatment. If you cannot return for blue light treatment on schedule, avoid sunlight and bright indoor light for at least 40 hours after LEVULAN KERASTICK topical solution has been applied.
- During blue light treatment, you will likely feel tingling, stinging, prickling, or burning of the treated areas.
What should I avoid during LEVULAN KERASTICK treatment?
After LEVULAN KERASTICK topical solution is applied to your skin you should avoid sunlight or bright indoor light (such as examination lights, operating room lights, tanning beds, or lights that are close to you) for 40 hours. During this time, the treated areas of your skin will become sensitive to light.
Exposure to light during this time may cause you to feel a burning or stinging sensation and may cause your treated lesions to become red or swollen. You should wear appropriate protective apparel such as a wide-brimmed hat, long sleeve shirt, and gloves to protect your treated skin from sunlight and other bright light.
Sunscreen will not protect the treated areas of your skin against sensitivity to light.
What are the possible side effects of LEVULAN KERASTICK?
LEVULAN KERASTICK may cause serious side effects, including:
- Temporary memory problems: Temporary memory problems have happened during treatment with LEVULAN KERASTICK in combination with BLU-U Blue Light Photodynamic Therapy Illuminator. You or your family members or caregiver should call your healthcare provider right away if you develop any problems with memory, confusion, or disorientation during treatment.
- Sensitivity to light
- Skin irritation. LEVULAN KERASTICK topical solution contains alcohol and may cause skin irritation if covered or bandaged for longer than 3 hours.
The common side effects of LEVULAN KERASTICK include:
Local skin reactions including redness, swelling, stinging and burning, scaling, crusting, oozing, pustules, welts, scabbing, itching, erosion, changes in skin color, bleeding, tenderness, changes in the sense of touch, and dryness.
These are not all the possible side effects of LEVULAN KERASTICK.
Call your doctor for medical advice about side effects.
Please read full Prescribing Information and Patient Information for LEVULAN KERASTICK and discuss any questions with your doctor.